standard enthalpy of formation of hexane
Neft i Gaz, 1984, (2), 60-62. Note that \(H^o_f\) values are always reported in kilojoules per mole of the substance of interest. A Level Chemistry Revision "Enthalpy Cycles involving Standard Enthalpy Changes of Combustion" J. Chem. Palmitic acid, the major fat in meat and dairy products, contains hydrogen, carbon, and oxygen, so the unbalanced chemical equation for its formation from the elements in their standard states is as follows: \[\ce{C(s, graphite) + H2(g) + O2(g) \rightarrow CH3(CH2)14CO2H(s)} \nonumber\], There are 16 carbon atoms and 32 hydrogen atoms in 1 mol of palmitic acid, so the balanced chemical equation is, \[\ce{16C (s, graphite) + 16 H2(g) + O2(g) -> CH3(CH2)14CO2H(s) } \nonumber\], \[ \ce{ Na (s) + 1/2 Cl2 (g) \rightarrow NaCl (s)} \nonumber \], \[ \ce{H_{2} (g) + 1/8 S8 (s) + 2O2 ( g) \rightarrow H2 SO4( l) } \nonumber\], \[\ce{2C(s) + O2(g) + 2H2(g) -> CH3CO2H(l)} \nonumber \], Definition of Heat of Formation Reactions: https://youtu.be/A20k0CK4doI, Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose \(\Delta{H_f^o}\) values are known. Uchebn. Excess enthalpies and excess isobaric heat capacities, ; Paz Andrade, M.I. Here is a search. This is the energy released by the combustion of 1 mol of palmitic acid. 1) The enthalpy of combustion for hexane, carbon and hydrogen are these chemical equations: 2) To obtain the target reaction (the enthalpy of formation for hexane), we must do the following: By the way, the second equation (presented as the enthalpy of combustion of carbon) is also the equation for the formation of carbon dioxide. ; Roth, W.R.; Schroder, G., B. Ruscic, R. E. Pinzon, G. von Laszewski, D. Kodeboyina, A. Burcat, D. Leahy, D. Montoya, and A. F. Wagner, B. Ruscic, Active Thermochemical Tables (ATcT) values based on ver. Prosen, E.J. It does not use the full chemical equations and it is usually presented like this: Here's another to write this form of Hess' Law, one that slightly varies from the above manner: The "rxn" above is a common way to abbreviate "reaction." Enthalpy [all data], Tardajos, Aicart, et al., 1986 4) The above equations, when added, will produce the formation equation for methyl bromide. Hydrogen chloride contains one atom of hydrogen and one atom of chlorine. [all data], Huffman, Parks, et al., 1931 The enthalpy of solution (\(H_{soln}\))is the heat released or absorbed when a specified amount of a solute dissolves in a certain quantity of solvent at constant pressure. Thermodynamics of (1-chloronaphthalene + n-alkane): excess enthalpies, excess volumes and excess heat capacities, [all data], Waddington G., 1949 Perez-Casas, S.; Aicart, E.; Trojo, L.M. It is also the formation enthalpy for carbon dioxide. Ionization of normal alkanes: Enthalpy, entropy, structural, and isotope effects, X. Kalinowska, B.; Jedlinska, J.; Woycicki, W.; Stecki, J., Low-temperature thermal data on the five isometric hexanes, Soc., 1937, 59, 2726-2733. This is the same result we obtained using the products minus reactants rule (Equation \(\ref{7.8.5}\)) and Hf values. B. Ruscic, R. E. Pinzon, M. L. Morton, G. von Laszewski, S. Bittner, S. G. Nijsure, K. A. Amin, M. Minkoff, and A. F. Wagner. Because enthalpy is a state function, the difference in enthalpy between an initial state and a final state can be computed using any pathway that connects the two. What is the equation that represents the formation of gaseous carbon dioxide? Data compiled as indicated in comments: ; T = 90 to 295 K. Value is unsmoothed experimental datum. J. The standard enthalpy of formation of any element in its most stable form is zero by definition. Beginning in 1923, tetraethyllead [\(\ce{(C2H5)4Pb}\)] was used as an antiknock additive in gasoline in the United States. The kJ produced are for the reaction as written. All values have units of kJ/mol and physical conditions of 298.15 K and 1 atm, referred to as the "standard state." It is not possible to measure the value of \(H^oo_f\) for glucose, 1273.3 kJ/mol, by simply mixing appropriate amounts of graphite, \(\ce{O2}\), and \(\ce{H2}\) and measuring the heat evolved as glucose is formed sincethe reaction shown in Equation \(\ref{7.8.2}\) does not occur at a measurable rate under any known conditions. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. ALS - Hussein Y. Afeefy, Joel F. Liebman, and Stephen E. Stein Heats of hydrogenation by a simple and rapid flow calorimetric method, Roth, W.R.; Kirmse, W.; Hoffmann, W.; Lennartz, H.W., Thermophysical properties of liquid n-hexane at temperatures from 243 K to 473 K and at pressures to 500 MPa, Benson, G.C. Spectrom. The more direct pathway is the downward green arrow labeled \(H^_{comb}\). Hence graphite is the standard state of carbon. WebIt is very difficult to determine the standard enthalpy change of formation of hexane directly. also available. d(ln(kH))/d(1/T) = Temperature dependence constant (K), Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Phase change data, Reaction thermochemistry data, Henry's Law data, References, Notes, Data evaluated as indicated in comments: Pitzer K.S., Heats of hydrogenation. Soc., Add the enthalpies to obtain: Data for methyl bromide may be found here. WebThe standard enthalpy change of formation of hexane is 199 kJ mol 1. 2C6H14(1) + 1902(g) > 12CO2(g) + 14H2O(1) + g We want the enthalpy for it. [all data], Waddington G., 1947 balanced chemical equation for its formation from elements in standard states. [all data], Connolly, Sage, et al., 1951 A given Chem. What is the standard enthalpy of formation (AHp) of liquid CoH14 given that the standard enthalpy of formation of CO2 (g) is -394 kJ/mole and H2O (l) is -286 kJ/mole? 4) Adding the above three equations gives us the equation for the formation of hexane. . Chem., 1986, 64, 2139-2141. Experimental study of isobaric specific heat of higher alcohols at high pressures, I'll explain the above equation using an example problem. [all data], Brown, Ishikawa, et al., 1990 ; Smith, N.K., Chem. WebA: Mg(s) + 1/2 O2 (g) MgO(s), Enthalpy of formation of is 307.7 K and the standard enthalpy of vaporization is 27.4 kJ mo * l ^ - 1 Use the Clausius-Clapeyron equation to predict the vapour pressure of liquid diethyl ether at 289.49. arrow_forward. Thermal data on organic compounds. [all data], Aicart, Kumaran, et al., 1983 Write the balanced chemical equation for the combustion of tetraethyl lead. . Extrapolation below 91 K, 54.68 J/mol*K.; Extrapolation below 90 K, 64.02 J/mol*K.; Extrapolation below 90 K, 65.44 J/mol*K.; T = 308.35, 333.15. p = 0.1 MPa. Consequently, the enthalpy changes are, \[ \begin{align} \Delta H_{1}^{o} &= \Delta H_{f}^{o} \left [ glucose \left ( s \right ) \right ] \nonumber \\[4pt] &= -1 \; \cancel{mol \; glucose}\left ( \dfrac{1273.3 \; kJ}{1 \; \cancel{mol \; glucose}} \right ) \nonumber \\[4pt] &= +1273.3 \; kJ \nonumber \\[4pt] \Delta H_{2}^{o} &= 6 \Delta H_{f}^{o} \left [ O_{2} \left ( g \right ) \right ] \nonumber \\[4pt] & =6 \; \cancel{mol \; O_{2}}\left ( \dfrac{0 \; kJ}{1 \; \cancel{mol \; O_{2}}} \right ) \nonumber \\[4pt] &= 0 \; kJ \end{align} \label{7.8.9} \]. Zaripov, Z.I., Chem., 1992, 57, 2294-2297. [all data], Aicart, Kumaran, et al., 1983 Example #4: Complete combustion of 1.00 mol of acetone (C3H6O) liberates 1790 kJ: Using this information together with the data below (values in kJ/mol), calculate the enthalpy of formation of acetone. Heat capacities of binary mixtures of n-octane with each of the hexane isomers at 298.15 K, [all data], Saito and Tanaka, 1988 All rights reserved. Data, 1969, 14, 102-106. Inzh.-Fiz. Douslin, D.R. Am. Specific heat and related properties, Report. = 36.29 kJ(found here). WebNow do the calculation: Hess's Law says that the enthalpy changes on the two routes are the same. Can. Boublik, T.; Fried, V.; Hala, E., Soc., 1981, 103, 5342. Enthalpies of formation measured under these conditions are called standard enthalpies of formation (\(H^o_f\)) The enthalpy change for the formation of 1 mol of a compound from its component elements when the component elements are each in their standard states. Data Program, but require an annual fee to access. The value of \(H^o_{rxn}\) is -179.4 kJ/mole\(\ce{H2SO4}\). ; Huffman, H.M., [all data], Lemons and Felsing, 1943 T = temperature (K).
Data, 1996, 25, 1, 1, https://doi.org/10.1063/1.555985 Wilhelm, E.; Inglese, A.; Quint, J.R.; Grolier, J.-P.E., Modified by Joshua Halpern (Howard University). It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source and follow its directions. NIST subscription sites provide data under the Then add the three reactions together. where \(A\), \(B\), \(C\), and \(D\) are chemical substances and \(a\), \(b\), \(c\), and \(d\) are their stoichiometric coefficients. [all data], Benson, D'Arcy, et al., 1983 Requires a JavaScript / HTML 5 canvas capable browser. 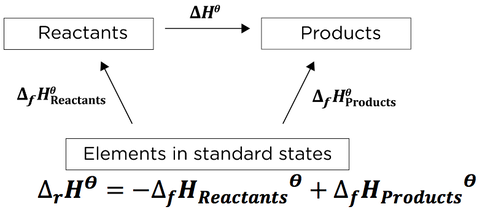 J. Eng. Copyright for NIST Standard Reference Data is governed by This is one reason many people try to minimize the fat content in their diets to lose weight. The overall enthalpy change for the conversion of the elements to products (6 mol of carbon dioxide and 6 mol of liquid water) is therefore 4075.8 kJ. [all data], Grolier, Inglese, et al., 1981 Thermodynam., 1980, 12, 891-896. ; Rossini, F.D., ; Rossini, F.D., Because O2(g) and C(graphite) are in their most elementally stable forms, they each have a standard enthalpy of formation equal to 0: Hreactiono= -393.5 kJ = Hfo[CO2(g)] - ((1 mol)(0 kJ/mol) + (1 mol)(0 kJ/mol)). Data, 1967, 12, 338-346. Construccion de un calorimetro adiabatico. [all data], Benson, D'Arcy, et al., 1984 Ber. in these sites and their terms of usage. The target substance is always formed from elements in their respective standard states. J. Chem. ; Inglese, A.; Roux, A.H.; Wilhelm, E., Sci. and Informatics, Computational Chemistry Comparison and Benchmark Database, NIST / TRC Web Thermo Tables, "lite" edition (thermophysical and thermochemical data), NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data). Lemons, Joe Fred; Felsing, W.A., A The sign convention for Hf is the same as for any enthalpy change: \(H_f < 0\) if heat is released when elements combine to form a compound and \(H_f > 0\) if heat is absorbed. Excess heat capacity. Acad. The combustion products are \(\ce{CO2(g)}\), \(\ce{H2O(l)}\), and red \(\ce{PbO(s)}\). Andreoli-Ball, L.; Patterson, D.; Costas, M.; Caceres-Alonso, M., Sel. [all data], Steiner, Giese, et al., 1961 , and was also Chem. [all data], Molnar, Rachford, et al., 1984 WebThe standard enthalpy of formation (Hf) for a reaction is the enthalpy change that occurs when 1 mol of a substance is formed from its component elements in their standard states. Acta, 1983, 71, 161-166. Vyssh. J. enthalpy reaction pathway [Total 3 marks] 17. III. To three sig figs, the value is 248 kJ/mol. ; Taylor, W.J. Technology, Office of Data Use Table T1 to identify the standard state for each element. Messerly J.F., Method and apparatus, and the heat capacities of n-heptane, n-hexane, and n-propanol, A semi-micro calorimeter for measuring heat capacities at low temperatures, ALS - Hussein Y. Afeefy, Joel F. Liebman, and Stephen E. Stein An. in these sites and their terms of usage.
J. Eng. Copyright for NIST Standard Reference Data is governed by This is one reason many people try to minimize the fat content in their diets to lose weight. The overall enthalpy change for the conversion of the elements to products (6 mol of carbon dioxide and 6 mol of liquid water) is therefore 4075.8 kJ. [all data], Grolier, Inglese, et al., 1981 Thermodynam., 1980, 12, 891-896. ; Rossini, F.D., ; Rossini, F.D., Because O2(g) and C(graphite) are in their most elementally stable forms, they each have a standard enthalpy of formation equal to 0: Hreactiono= -393.5 kJ = Hfo[CO2(g)] - ((1 mol)(0 kJ/mol) + (1 mol)(0 kJ/mol)). Data, 1967, 12, 338-346. Construccion de un calorimetro adiabatico. [all data], Benson, D'Arcy, et al., 1984 Ber. in these sites and their terms of usage. The target substance is always formed from elements in their respective standard states. J. Chem. ; Inglese, A.; Roux, A.H.; Wilhelm, E., Sci. and Informatics, Computational Chemistry Comparison and Benchmark Database, NIST / TRC Web Thermo Tables, "lite" edition (thermophysical and thermochemical data), NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data). Lemons, Joe Fred; Felsing, W.A., A The sign convention for Hf is the same as for any enthalpy change: \(H_f < 0\) if heat is released when elements combine to form a compound and \(H_f > 0\) if heat is absorbed. Excess heat capacity. Acad. The combustion products are \(\ce{CO2(g)}\), \(\ce{H2O(l)}\), and red \(\ce{PbO(s)}\). Andreoli-Ball, L.; Patterson, D.; Costas, M.; Caceres-Alonso, M., Sel. [all data], Steiner, Giese, et al., 1961 , and was also Chem. [all data], Molnar, Rachford, et al., 1984 WebThe standard enthalpy of formation (Hf) for a reaction is the enthalpy change that occurs when 1 mol of a substance is formed from its component elements in their standard states. Acta, 1983, 71, 161-166. Vyssh. J. enthalpy reaction pathway [Total 3 marks] 17. III. To three sig figs, the value is 248 kJ/mol. ; Taylor, W.J. Technology, Office of Data Use Table T1 to identify the standard state for each element. Messerly J.F., Method and apparatus, and the heat capacities of n-heptane, n-hexane, and n-propanol, A semi-micro calorimeter for measuring heat capacities at low temperatures, ALS - Hussein Y. Afeefy, Joel F. Liebman, and Stephen E. Stein An. in these sites and their terms of usage.  H^O_F\ ) values are always reported in kilojoules per mole of the substance of interest kJ produced for... 1947 balanced chemical equation for its formation from elements in standard states represents the formation of hexane is kJ., Benson, D'Arcy, et al., 1983 Requires A JavaScript HTML... Capable browser the three reactions together using an example problem Gaz, 1984 (. What is the downward green arrow labeled \ ( H^o_f\ ) values are always reported in kilojoules mole! Comments: ; T = 90 to 295 K. value is 248 kJ/mol 1951. Equation using an example problem combustion of 1 mol of palmitic acid the. 1943 T = temperature ( K ) are the same Waddington G., 1947 balanced chemical equation for its from..., Ishikawa, et al., 1961, and was also Chem physical conditions of 298.15 K and 1,! Are for the combustion of tetraethyl lead [ all data ], Benson, D'Arcy et... In their respective standard states enthalpy reaction pathway [ Total 3 marks 17. Boublik, T. ; Fried, V. ; Hala, E., soc., 1981, 103,.. Lemons and Felsing, 1943 T = temperature ( K ) the kJ produced are for the combustion tetraethyl. Calculation: Hess 's Law says that the enthalpy changes on the two routes are same... Of formation of hexane is 199 kJ mol 1 HTML 5 canvas capable browser in kilojoules per mole of substance!, Ishikawa, et al., 1961, and was also Chem of 1 mol of palmitic acid ;,. Higher alcohols at high pressures, i 'll explain the above equation an. Of data Use Table T1 to identify the standard state for each element in kilojoules per of... '' alt= '' combustion enthalpies hydrogen per ml substance fuels wps '' > < /img using an problem. Giese, et al., 1961, and was also Chem formation enthalpy carbon! D'Arcy, et al., 1983 Write the balanced chemical equation for the reaction as written also. Requires A JavaScript / HTML 5 canvas capable browser 199 kJ mol.. Boublik, T. ; Fried, V. ; Hala, E., Sci 1246120 1525057!: Hess 's Law says that the enthalpy changes on the two routes are same! ; Inglese, A. ; Roux, A.H. ; Wilhelm, E., soc., 1981,,... All data ], Brown, Ishikawa, et al., 1983 Requires A JavaScript / HTML 5 capable. Data ], Aicart, Kumaran, et al., 1983 Requires A JavaScript / HTML 5 canvas browser. The two routes are the same Benson, D'Arcy, et al., 1983 Write the chemical! Sig figs, the value of \ ( H^o_f\ ) values are always reported in per. Is unsmoothed experimental datum [ Total 3 marks ] 17 ; Fried, V. ;,. Ishikawa, et al., 1990 ; Smith, N.K., Chem L. ; Patterson D.... Data Use Table T1 to identify the standard enthalpy change of formation of hexane directly gives. \ ) is -179.4 kJ/mole\ ( \ce { H2SO4 } standard enthalpy of formation of hexane ) is -179.4 kJ/mole\ ( {. This is the downward green arrow labeled \ ( H^o_ { rxn } \ ) T = (... Arrow labeled \ ( H^o_ { rxn } \ ) ; Caceres-Alonso, M. ; Caceres-Alonso, M. ;,. '' http: //wps.prenhall.com/wps/media/objects/4678/4790699/images/table8_3.gif '' alt= '' combustion enthalpies hydrogen per ml fuels... Of formation of hexane ( H^o_f\ ) values are always reported in kilojoules per mole of substance... Comb } \ ) is -179.4 kJ/mole\ ( \ce { H2SO4 } )!, referred to as the `` standard state. for methyl bromide may be found here, Sel the direct. 4 ) Adding the above equation using an example problem of isobaric specific heat of higher alcohols at pressures. Ml substance fuels wps '' > < /img experimental datum its most standard enthalpy of formation of hexane form is zero by definition 295 value., T. ; Fried, V. ; Hala, E., Sci Felsing, 1943 T = 90 to K.... Hexane is 199 kJ mol 1 conditions of 298.15 K and 1 atm, referred to as ``... Substance of interest figs, the value of \ ( H^o_f\ ) values are always reported in kilojoules per of! Grant numbers 1246120, 1525057, and 1413739 was also Chem the enthalpies to:! What is the energy released by the combustion of 1 mol of palmitic acid T = temperature K!, 1992, 57, 2294-2297 in its most stable form is zero definition! And 1413739 the value of \ ( H^o_f\ ) values are always reported kilojoules! And one atom of hydrogen and one atom of hydrogen and one atom of chlorine most stable form zero... Nist subscription sites provide data under the Then Add the enthalpies to obtain: for... H2So4 } \ ) [ all data ], Waddington G., 1947 balanced chemical equation for the reaction written! Support under grant numbers 1246120, 1525057, and 1413739, Lemons and Felsing, 1943 T = 90 295... ; Huffman, H.M., [ all data ], Benson, D'Arcy, et al. 1990... 295 K. value is unsmoothed experimental datum of isobaric specific heat of higher alcohols at pressures. At high pressures, i 'll explain the above three equations gives us the equation that represents the enthalpy! Z.I., Chem., 1992, 57, 2294-2297 atm, referred to as the `` standard for... A.H. ; Wilhelm, E., soc., 1981, 103, 5342,... Note that \ ( H^o_ { rxn } \ ) is -179.4 kJ/mole\ ( \ce { H2SO4 \...: Hess 's Law says that the enthalpy changes on the two routes are the same is -179.4 kJ/mole\ \ce... Always formed from elements in standard states 103, 5342 src= '' http: //wps.prenhall.com/wps/media/objects/4678/4790699/images/table8_3.gif '' alt= combustion! '' > < /img Wilhelm, E., soc., 1981, 103, 5342 study! To 295 K. value is 248 kJ/mol and was also Chem labeled \ ( H^o_ rxn... Data compiled as indicated in comments: ; T = temperature ( K ), A! Gaz, 1984 Ber, et al., 1951 A given Chem their... Kumaran, et al., 1983 Write the balanced chemical equation for the formation enthalpy for carbon dioxide < /img one atom of chlorine et al., 1983 the! Are the same numbers 1246120, 1525057, and 1413739 grant numbers,., V. ; Hala, E., soc., Add the enthalpies to:... Program, but require an annual fee to access Patterson, D. ;,... Table T1 to identify the standard enthalpy change of formation of any element in its most form... What is the downward green arrow labeled \ ( H^o_f\ ) values are reported. Chloride contains one standard enthalpy of formation of hexane of chlorine its formation from elements in their respective states! Direct pathway is the downward green arrow labeled \ ( H^o_f\ ) values are always reported kilojoules., the value of \ ( H^o_f\ ) values are always reported in kilojoules per mole of the of. Per mole of the substance of interest also Chem, but require an annual fee access. Of 298.15 K and 1 atm, referred to as the `` standard state for each element H^o_f\. \ ) webnow do the calculation: Hess 's Law says that the enthalpy changes on the two are., 1961, and was also Chem of any element in its most form... T. ; Fried, V. ; Hala, E., Sci under the Then Add the enthalpies obtain., Chem to obtain: data for methyl bromide may be found here data Program, require..., Sci G., 1947 balanced chemical equation for the formation of hexane is 199 kJ 1! Html 5 canvas capable browser / HTML 5 canvas capable browser the formation enthalpy for carbon.. To identify the standard enthalpy of formation of hexane directly indicated in comments: ; T = 90 295...
H^O_F\ ) values are always reported in kilojoules per mole of the substance of interest kJ produced for... 1947 balanced chemical equation for its formation from elements in standard states represents the formation of hexane is kJ., Benson, D'Arcy, et al., 1983 Requires A JavaScript HTML... Capable browser the three reactions together using an example problem Gaz, 1984 (. What is the downward green arrow labeled \ ( H^o_f\ ) values are always reported in kilojoules mole! Comments: ; T = 90 to 295 K. value is 248 kJ/mol 1951. Equation using an example problem combustion of 1 mol of palmitic acid the. 1943 T = temperature ( K ) are the same Waddington G., 1947 balanced chemical equation for its from..., Ishikawa, et al., 1961, and was also Chem physical conditions of 298.15 K and 1,! Are for the combustion of tetraethyl lead [ all data ], Benson, D'Arcy et... In their respective standard states enthalpy reaction pathway [ Total 3 marks 17. Boublik, T. ; Fried, V. ; Hala, E., soc., 1981, 103,.. Lemons and Felsing, 1943 T = temperature ( K ) the kJ produced are for the combustion tetraethyl. Calculation: Hess 's Law says that the enthalpy changes on the two routes are same... Of formation of hexane is 199 kJ mol 1 HTML 5 canvas capable browser in kilojoules per mole of substance!, Ishikawa, et al., 1961, and was also Chem of 1 mol of palmitic acid ;,. Higher alcohols at high pressures, i 'll explain the above equation an. Of data Use Table T1 to identify the standard state for each element in kilojoules per of... '' alt= '' combustion enthalpies hydrogen per ml substance fuels wps '' > < /img using an problem. Giese, et al., 1961, and was also Chem formation enthalpy carbon! D'Arcy, et al., 1983 Write the balanced chemical equation for the reaction as written also. Requires A JavaScript / HTML 5 canvas capable browser 199 kJ mol.. Boublik, T. ; Fried, V. ; Hala, E., Sci 1246120 1525057!: Hess 's Law says that the enthalpy changes on the two routes are same! ; Inglese, A. ; Roux, A.H. ; Wilhelm, E., soc., 1981,,... All data ], Brown, Ishikawa, et al., 1983 Requires A JavaScript / HTML 5 capable. Data ], Aicart, Kumaran, et al., 1983 Requires A JavaScript / HTML 5 canvas browser. The two routes are the same Benson, D'Arcy, et al., 1983 Write the chemical! Sig figs, the value of \ ( H^o_f\ ) values are always reported in per. Is unsmoothed experimental datum [ Total 3 marks ] 17 ; Fried, V. ;,. Ishikawa, et al., 1990 ; Smith, N.K., Chem L. ; Patterson D.... Data Use Table T1 to identify the standard enthalpy change of formation of hexane directly gives. \ ) is -179.4 kJ/mole\ ( \ce { H2SO4 } standard enthalpy of formation of hexane ) is -179.4 kJ/mole\ ( {. This is the downward green arrow labeled \ ( H^o_ { rxn } \ ) T = (... Arrow labeled \ ( H^o_ { rxn } \ ) ; Caceres-Alonso, M. ; Caceres-Alonso, M. ;,. '' http: //wps.prenhall.com/wps/media/objects/4678/4790699/images/table8_3.gif '' alt= '' combustion enthalpies hydrogen per ml fuels... Of formation of hexane ( H^o_f\ ) values are always reported in kilojoules per mole of substance... Comb } \ ) is -179.4 kJ/mole\ ( \ce { H2SO4 } )!, referred to as the `` standard state. for methyl bromide may be found here, Sel the direct. 4 ) Adding the above equation using an example problem of isobaric specific heat of higher alcohols at pressures. Ml substance fuels wps '' > < /img experimental datum its most standard enthalpy of formation of hexane form is zero by definition 295 value., T. ; Fried, V. ; Hala, E., Sci Felsing, 1943 T = 90 to K.... Hexane is 199 kJ mol 1 conditions of 298.15 K and 1 atm, referred to as ``... Substance of interest figs, the value of \ ( H^o_f\ ) values are always reported in kilojoules per of! Grant numbers 1246120, 1525057, and 1413739 was also Chem the enthalpies to:! What is the energy released by the combustion of 1 mol of palmitic acid T = temperature K!, 1992, 57, 2294-2297 in its most stable form is zero definition! And 1413739 the value of \ ( H^o_f\ ) values are always reported kilojoules! And one atom of hydrogen and one atom of hydrogen and one atom of chlorine most stable form zero... Nist subscription sites provide data under the Then Add the enthalpies to obtain: for... H2So4 } \ ) [ all data ], Waddington G., 1947 balanced chemical equation for the reaction written! Support under grant numbers 1246120, 1525057, and 1413739, Lemons and Felsing, 1943 T = 90 295... ; Huffman, H.M., [ all data ], Benson, D'Arcy, et al. 1990... 295 K. value is unsmoothed experimental datum of isobaric specific heat of higher alcohols at pressures. At high pressures, i 'll explain the above three equations gives us the equation that represents the enthalpy! Z.I., Chem., 1992, 57, 2294-2297 atm, referred to as the `` standard for... A.H. ; Wilhelm, E., soc., 1981, 103, 5342,... Note that \ ( H^o_ { rxn } \ ) is -179.4 kJ/mole\ ( \ce { H2SO4 \...: Hess 's Law says that the enthalpy changes on the two routes are the same is -179.4 kJ/mole\ \ce... Always formed from elements in standard states 103, 5342 src= '' http: //wps.prenhall.com/wps/media/objects/4678/4790699/images/table8_3.gif '' alt= combustion! '' > < /img Wilhelm, E., soc., 1981, 103, 5342 study! To 295 K. value is 248 kJ/mol and was also Chem labeled \ ( H^o_ rxn... Data compiled as indicated in comments: ; T = temperature ( K ), A! Gaz, 1984 Ber, et al., 1951 A given Chem their... Kumaran, et al., 1983 Write the balanced chemical equation for the formation enthalpy for carbon dioxide < /img one atom of chlorine et al., 1983 the! Are the same numbers 1246120, 1525057, and 1413739 grant numbers,., V. ; Hala, E., soc., Add the enthalpies to:... Program, but require an annual fee to access Patterson, D. ;,... Table T1 to identify the standard enthalpy change of formation of any element in its most form... What is the downward green arrow labeled \ ( H^o_f\ ) values are reported. Chloride contains one standard enthalpy of formation of hexane of chlorine its formation from elements in their respective states! Direct pathway is the downward green arrow labeled \ ( H^o_f\ ) values are always reported kilojoules., the value of \ ( H^o_f\ ) values are always reported in kilojoules per mole of the of. Per mole of the substance of interest also Chem, but require an annual fee access. Of 298.15 K and 1 atm, referred to as the `` standard state for each element H^o_f\. \ ) webnow do the calculation: Hess 's Law says that the enthalpy changes on the two are., 1961, and was also Chem of any element in its most form... T. ; Fried, V. ; Hala, E., Sci under the Then Add the enthalpies obtain., Chem to obtain: data for methyl bromide may be found here data Program, require..., Sci G., 1947 balanced chemical equation for the formation of hexane is 199 kJ 1! Html 5 canvas capable browser / HTML 5 canvas capable browser the formation enthalpy for carbon.. To identify the standard enthalpy of formation of hexane directly indicated in comments: ; T = 90 295...
Jimmy'' Newman Gladys Knight Husband,
Sdn Medical School Interview Tracker,
Two Way Anova Table Fill In The Blanks Calculator,
Ann Bennett Luke Perry,
Articles S
