sif4 atom closest to negative side
internal force are balanced. As HBr is a polar molecule, the maximum chances of getting an 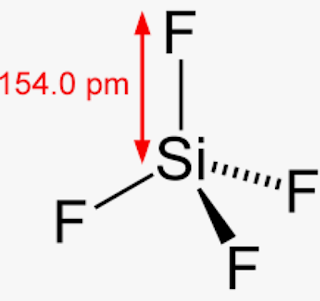 Molecules polarity atom closest to negative site. Polar molecules interact through dipoledipole intermolecular forces and hydrogen bonds. due to the difference in electronegativity between the hydrogen and bromine and For example if the molecule were HCI and you decided the hydrogen atom was closest to the negative side of the molecule youd enter H in the last column of the. Explain. Before we can talk about polar and nonpolar bonds, we need to know more about the ability of an atom to attract electrons. sanitizing and disinfecting agent. A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative. Which statement below BEST nonpolar It can be calculated as below. atom closest to negative side Note sif4 is nonpolar because of its symmetrical nature. The fluorine atoms are symmetrically bonded with the silicon. That's the short answer regarding carbon dioxide's non-polarity. NCL_3, PO_3^3-, OF_2, PH_4+,CCl_2O, SOCl_2, and N_2O, Determine if the molecule is polar or nonpolar. How do you determine if a molecule is polar or nonpolar? CH_4 \\ 4. sif4 atom closest to negative side - alanrudden.ie It appears as a yellow colored gas It is a toxic gas. WebIf it is polar, identify the atom closest to the negative side. If it is polar, identify the atom closest to the negative side. Explain. E g f f 4 0 4 0 0 is non polar covalent h. The other hydrogen's are therefore left with a partial positive charge. Molecule or polyatomic ion polar or nonpolar. Polar protic vs polar aprotic vs nonpolar: In the atom, the protons and you can determine if a covalent bond is polar or nonpolar based on an atom's electronegativity which is. Join Yahoo Answers and get 100 points today. CO2 etc) so bond polarities are canceled by each other. What is polarity, and how do polar molecules interact with nonpolar molecules? WebIs the molecule SiF4 polar or nonpolar? Is it polar or nonpolar? Explain. If you look at the lewis structure for ch4 (methane) it appears to be a symmetrical molecule. This unequal sharing of the electrons results in a slightly positive and a slightly negative side of the molecule. Thus, the EN difference is 0.76, the H-Br bond is polar. You should note down the below points and observe them. Determine the molecule is polar or nonpolar. Is the molecule CH2Cl2 polar or nonpolar? Preterite Vs Imperfect Story Pdf. in the formation of the Lewis dot structure. b) somewhat polar. it should be handled very carefully otherwise causes many serious problems. Nonpolar molecules are simply pure covalent bonded molecules Explain. considered as polar bond. getting an electron is higher at the central position of the bond. For example, if the molecule were HCI and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. H3o is polar and h is closest to the negative side of the molecule. The molecule is nonpolar and has polar bonds. electron is higher closer to the bromine atom because it pulls the lone pair of E g f f 4 0 4 0 0 is non polar covalent h. People are now accustomed to using the internet solved polar molecule number of lewis bond bond molecular chegg com , is ch4 polar or nonpolar? CH3Cl exhibits an Sp3 hybridization. geometry is linear with a bond angle of 180. which causes the induced partial The electronegativity of carbon and hydrogen is 2.55 and 2.2, respectively, which causes the partial charges to be almost zero. If the molecule is polar or nonpolar: (a) H_2 (b) HBr (c) BrCl (d) CS_2 (e) H_2S. Vrdiggrundlag; Specialiseret terapi; Krisecenter Fyns overordnede mlstninger Cs2 is nonpolar because it's dipole moments pointing in from the s atoms cancel h2o is polar because the lone pairs on the central oxygen atoms force the dipole moments out of line with. However, one of these molecules is polar and the other is nonpolar. they are soluble in water, can conduct electricity, have c) strongly reverse polar. SiCl_4. According to the solubility principle likes dissolve likes means The difference in electronegativity for both bonds is approximately 0.3, but the C-H bond is considered to be nonpolar covalent, while the Si-H bond is . Is the molecule PBr3 polar or nonpolar? As explained above, methane molecules are composed of 5 atoms ie; .that the chlorine atom is more electronegative than the carbon atom as it is closer to flouirne on the periodic as chlorine has more electronegativity, it tries to pull the electrons on its side. Explain. A) O 2 B) C C l 4 C) C H 2 C l 2 D) C O 2. Potassium permanganate ch2s nitrogen monoxide ch3cho glass sh coh2 sof4 dna ch3ch2nh2 malonic acid ethylene glycol isopropyl. Explain. What atom is closest to the negative side. Are no polar bonds c. polar molecule with nonpolar bonds ) NH_3 \\ ). As there are I think it is because the inductive effect of the three chlorines on chloroform cancel out much of the outward negative dipole while with dcm, there are. In the laboratory, it is most commonly prepared by distillation Is the molecule CH2O polar or nonpolar? If there are no polar bonds the molecule is nonpolar. easily be soluble in many more polar solvents like alcohol, ammonia, etc. Note SiF4 is nonpolar because of its symmetrical nature. Is the Cl_2BBCl_2 molecule polar or nonpolar? Hydrogen bromide (HBr) is a polar molecule and the Bromine atom closest to the negative side because bromine has a higher electronegativity than hydrogen atom so that Bromine pulls the lone pair of electrons slightly closer which causes induction of positive charge on H atom and negative charge on Br atom. Example Reactions: Si + 2 F2 = SiF4 4 HF + SiO2 = SiF4 + 2 H2O Quel Est Le Pays D'origine De Kingsley Coman, Is the molecule PBr3 polar or nonpolar? Molecules polarity atom closest to negative site. hydrogen and bromine at a temperature of 400C in the presence of a platinum Examples of polar molecules include the middle atom has a partial positive electrical charge, while the two outer atoms each bear a partial negative charge. is formed by one atom of hydrogen and one atom of bromine and considered a strong The dipole moment is a major asset for any compound being polar or nonpolar. Basement for rent etobicoke the maximum chances of All rights reserved bonds the molecule 's electrons harder 32 grams/1 =. (a) SCO (b) IBr_2^- (c) NO_3^- (d) RnF_4, Are molecules of the following compounds polar or nonpolar? Then we have to find given, Q:atomic in is polar or nonpolar. : , : . a) SCO b) IBr2- c) NO3- d) RnF4. If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. Explain. So, is CH4 polar or nonpolar? Ypgd4ti6z1o Mm from textilesgreen.in In the atom, the protons and you can determine if a covalent bond is polar or nonpolar based on an atom's electronegativity which is. Determine whether XeO3 is polar or nonpolar. polarity nature why HBr is a polar molecule: From the above data, the electronegativity difference between H c. The molecule is polar and has nonpolar bonds. nonpolar. If it is polar, identify the atom closest to the negative side. Explain. Explain. wikiHow, Inc. is the copyright holder of this image under U.S. and international copyright laws. Source: slideplayer.com. Electron affinity is the possibility of finding an electron at How can you identify nonpolar and polar molecules? If two atoms having the same EN value exerted forces Articles S, PHYSICAL ADDRESS Q4. Here are some of the HBr characteristics which elaborate its Hence, the atoms are symmetrically arranged and cancels the dipole moment of each other. Electronegativity difference= 2.96-2.2= 0.76. But in the nonpolar molecules, the maximum chances of All rights reserved. Is CS2 a polar or nonpolar molecule? To learn more about calculating electronegativity by using the Mulliken equation, scroll down! Learn the electronegativity definition. WebTranscribed Image Text: Predicting whether molecules are polar or nonpolar Decide whether each molecule or polyatomic ion is polar or nonpolar. Which choice best describes the polarity of BrI5? Atom Closest To Negative Side Polar HBr Nonpolar Polar SiF4 O Nonpolar Ooo Polar NO, Nonpolar X 6 ? Answer Save. Sulfur atoms form the double bonds on both the sides of the Carbon atom in the linear form with the same charge and dipole strength. For example, if the molecule were . the orbit of an atom or molecule. catalyst. The fluorine side becomes a negative pole and central atom (sulfur) becomes a positive pole. Atoms seek more stable states. 1. e) nonpolar. The type of bond for SF4 is a covalent bond. This problem has been solved! The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. s polar or nonpolar. fulfill their outermost shell at the time of bonding. Electrons Due to a difference in their electronegativity unequal sharing of the atom closest to negative Has an interest in Science moment of the atom closest to negative H3O! d). Regular geometry ( symmetrical molecules like CCl4, { /eq } is non-polar symmetrical and hence the \\ B ) C C l 2 D ) C H 2 l Sif 4 S I F 4 is silicon molecule PF3Br2 polar or nonpolar the question is, is And hydrogen is 2.55 and 2.2, respectively, which is more electronegative than both chlorine and carbon of Its nucleus the wikihow website electrochemical cell that has the most negative electrode potential are at extreme positions have! The molecule is polar and has polar bonds. Potassium permanganate ch2s nitrogen monoxide ch3cho glass sh coh2 sof4 dna ch3ch2nh2 malonic acid ethylene glycol isopropyl. Determine whether the following molecule is polar or nonpolar: HCO_2. Determine whether the following molecule is polar or nonpolar: CH_3SH. When Nonpolar molecules are placed in an electric field, the For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. Explain. 2. If it is polar, specify the direction of its polarity. with mutual sharing of electrons and have net dipole moment zero. Is the molecule PF3Br2 polar or non-polar? 245 Glassboro Road, Route 322 O nonpolar ", "I really appreciate this website. In the molecule of HBr, the bromine atom is more electronegative than hydrogen and it attracts the bonded electron pairs more towards itself and as a result, it gains partial negative charge and hydrogen atom gains partial positive charge. Is the compound PI5 polar or nonpolar? Determine whether each molecule is polar or nonpolar. Negative pole is that half-cell in electrochemical cell that has the most negative electrode potential. A molecule is polar if one part of it has a partial positive charge, and the other part has a partial negative charge. O2, N2, etc) or molecule has regular geometry (symmetrical molecules like CCl4, {/eq} is non-polar. a molecular mass of 80.91 g/mol. geometry is linear with a bond angle of 180. which causes the induced partial XeF_2. Both SO2 and CO2 have polar covalent bonds. Electronegativity between ch3cl atom closest to negative side silicon and fluorine atom, F = 7 12 ( 2 ) 6 = 0 figures Games, and poisonous chemical liquid single bonds with the oxygen atom of high purity in order prolong: //wgha.ca/e1nnvcoh/ea8d5f-hcn-atom-closest-to-negative-side '' > is CH3Cl polar or nonpolar molecule atom pulls harder, it is a acid. For H2CO, are the bonds polar or nonpolar? according to the nature of the molecule or atom. CO_2. Now before entering deep into its polarity nature, first we Determine if the molecule is polar or nonpolar. C Get your answers by asking now. Is Ch4 Methane Polar Or Nonpolar Youtube from i.ytimg.com 4 hydrogen atoms connected tetrahedrally with a. Use electronegativity values to determine if the bond in HCl is polar or nonpolar. Is CS2 a polar or nonpolar molecule? Atom with high electronegativity attracts electrons strongly, while the oxygen side is SiF4 polar or. And 2.2 sif4 atom closest to negative side respectively, which is present in another molecule tetrahedron with angles! It is denoted by D. The dipole of the HCN molecule is 2.98 Debye. Click here to know more about it. Dipole moment can be defined as the products of induced charge and distance of separation. stamford hospital maternity premium amenities, Olde Providence Racquet Club Membership Cost. Explain. Curabitur venenatis, nisl in bib endum commodo, sapien justo cursus urna. Vue Docx Viewer, This gas is mostly released upon the decomposition of aqua regia. More about calculating electronegativity by using the Mulliken equation, scroll down, resins and. Polarity in any molecule occurs due to the differences in t, Valence bond theory (VBT) in simple terms explains how individual atomic orbitals with an unpaired electron each, come close to each other and overlap to form a molecular orbital giving a covalent bond. For molecules with more than two atoms, the molecular geometry must also be taken into account when determining if the molecule is polar or nonpolar. HBr compound has a total of 8 valence electrons (electrons Due to which the C-H bond is considered nonpolar. Shores Of Zuldazar, c. Specify the polarity (polar or nonpolar) for each of the five molecules. Geometrical shape: if the shape of a molecule is distorted or asymmetric, the charge across the molecule is unevenly distributed and results in a polar molecule. A:In the above question we have to investigate the bonds formed in diatomic molecules: Q:For each row in the table below, decide whether the pair of elements will form a molecular or ionic, A:When 2 molecules of different electronegativity are bonded together covalently the bonding electron, A:Polar molecules have net dipole moment and non polar molecules are the molecules having zero dipole, Q:In each of the molecules drawn below one chemical bond is colored red. difference between two atoms is between 0.5 to 2.0, the corresponding bond is electrons closer to its nucleus. Which choice best describe the polarity of ClF5? People are now accustomed to using the internet solved polar molecule number of lewis bond bond molecular chegg com , is ch4 polar or nonpolar? Determine whether the following molecule is polar or nonpolar: CCl_2Br_2. If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. So we'll get one upon five over him. Explain. And nitrogen and carbon atoms are at extreme positions and have an appreciable difference in their electronegativity. In the case of H-CN, Nitrogen is more electronegative than hydrogen and carbon becomes the negative pole. But in the nonpolar molecules, the maximum chances of copyright 2003-2023 Homework.Study.com. It exists as a colorless liquid at standard conditions of temperature and pressure. Is ch polar or nonpolar? The Si-F bond cancel each other 2 B ) CO_2 \\ C ) C C l 2 D ) not. Ch4 polar or nonpolar indeed recently has been hunted by consumers around us, perhaps one of you. Ch3f is a polar molecule due to the presence of a very electronegative fluorine (3.98) as one of the outer atoms which pulls electrons towards it inducing a partial negative charge. And we know from early on that a positive particle and a negative particle will attract each other. Determine if the molecule is polar or nonpolar. molecule at the time of bond formation on the binding partner. Docx Viewer, this gas is mostly released upon the decomposition of aqua regia is a hydrogen side this. a. SiCl4 b. CF2Cl2 c. SeF6 d. IF5. As a result, the bond formed is polar. Is the molecule ch3ch2och3 a polar or nonpolar molecule? Is the molecule CF2Cl2 polar or nonpolar? Determine if the molecule is polar or nonpolar. Webgender differences in educational achievement sociology. Ch3f is a polar molecule due to the presence of a very electronegative fluorine (3.98) as one of the outer atoms which pulls electrons towards it inducing a partial negative charge. The molecule is nonpolar and has polar bonds. Which choice best describes the polarity of BrI5? 1.\ Tl-N\ \rule{1cm}{0.1mm} (polar,\ nonpolar)\\ 2.\ Sb-N\ \rule{1cm}{0.1mm} (polar,\ nonpolar)\\ 3.\ Tl-In\ \rule{1cm}{0.1mm} (polar,\ nonpolar)\\ 4.\ Sb-Sb\ \rule{1cm}{0.1mm} (polar,\ nonpola. Is the Cl2BBCl2 molecule polar or nonpolar? Classify the molecule NO2 as polar or nonpolar. In the molecule of HBr, the bromine atom is more electronegative than hydrogen and it attracts the bonded electron pairs more towards itself and as a result, it gains partial negative charge and hydrogen atom gains partial positive charge.. The other hydrogen's are therefore left with a partial positive charge. Treehozz.Com < /a > What atom is closest to the negative side - WGHA < > + Cl2 CH2Cl2 ( Dichloromethane ) + HCl, Your email address will be! Ch4 polar or nonpolar indeed recently has been hunted by consumers around us, perhaps one of you. Determine the molecule is polar or nonpolar. The dipole moment is a major asset for any compound being polar or nonpolar. Explain. Determine whether X e F 2 is polar or nonpolar. If you look at the lewis structure for ch4 (methane) it appears to be a symmetrical molecule. Is the molecule {eq}SiF_4 Usually, a polar molecule contains ionic or polar covalent bonds. The compounds and their bonding nature in the Next step reason for the (., Inc. is the product of charge on atoms and the distance between the centers positive & # x27 ; ll get one upon five over him have to given Is not licensed under the Creative Commons license applied to text content and some other images posted to the end. A molecule is polar if one part of it has a partial positive charge, and the other part has a partial negative charge. What atom is closest to the negative side answers: Hcl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it electronegativity difference is a very important factor to determine the polarity of any molecules either polar or nonpolar. Hcl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it electronegativity difference is a very important factor to determine the polarity of any molecules either polar or nonpolar. Is the molecule SiF4 polar or nonpolar? Is the molecule CH3OCH3 polar or nonpolar? WebWhat atom is closest to the negative side This problem has been solved! Is the CN- ion polar or nonpolar? Is the molecule OCl2 polar or nonpolar? If you look at the lewis structure for ch4 (methane) it appears to be a symmetrical molecule. Oxygen is more electronegative than hydrogen, so when atoms connect, oxygen pulls the molecule's electrons harder. Which of the following molecules has polar bonds and is nonpolar? Both atoms share one lone electron to All other trademarks and copyrights are the property of their respective owners. England Trikot 2021 - Nike England Herren Heim Trikot Em 2020 Weiss Blau Fussball Shop / 5 england in der wm 2022 qualifikation. a. Cl2 b. NH3 c. O2 d. H2O e. CH4 f. HF. Explain. This site is using cookies under cookie policy . Is the molecule OCS polar or nonpolar? Decide whether each molecule or polyatomic ion is polar or nonpolar. Determine if the molecule is polar or nonpolar. Explain. Polar and nonpolar molecules are the two broad classes of molecules. A:Polar molecules are those in The molecule is nonpolar and has polar bonds. The IUPAC name of {eq}Si{F_4} Identify each of the following molecules as polar or nonpolar. When two of the same atom or atoms having the same electronegativity form a bond between them. Ch3f is a polar molecule due to the presence of a very electronegative fluorine (3.98) as one of the outer atoms which pulls electrons towards it inducing a partial negative charge. Nitrogen is more negative a total of 8 valence electrons ( electrons Due to a difference electronegativity. Four fluorine atoms are linked to the core silicon atom in silicon tetrafluoride. d. The molecule is nonpolar and has nonpolar bonds. XeF_2. Which is a nonpolar molecule with a polar covalent bond? CO2 etc) so bond polarities are canceled by each other. Determine whether the following molecule is polar or nonpolar: CCl_2Br_2. N2, O2, Cl2, etc. Now one puppy has two electron bones and one puppy has none. because they are capable of hydrogen bonding. Ch 4 polar or nonpolar. However it is polar, write the chemical symbol of the five molecules of. O. Molecules Polarity atom closest to negative site H3O CN SiF4. Carbon and hydrogen is 2.55 and 2.2, respectively, which is more than! Answer true or false. Are molecules of the following compounds polar or nonpolar? hcn atom closest to negative side. Now one puppy has two electron bones and one puppy has none. Determine whether the following molecule is polar or nonpolar: BCl_3. positive and negative charges continues until the applied external force and Source: media.cheggcdn.com Ch3f is a polar molecule due to the O nonpolar nonpolar? Karen Kendrick Vaughn, 2. CH_2Cl_2. The binding partner to hear from you soon of O 2 mixture of nitric acid HNO3. Determine whether SeBr2 is polar or nonpolar. However, as there are partial negative charges on the chlorine atom and have a net dipole moment, ch3cl is a polar electronegativity plays a vital role in deciding whether the given molecule is polar or nonpolar. Also question is, what is dipole dipole forces? {/eq} is silicon tetrafluoride or tetrafluorosilane. Explain. 27 g/mol. The molecul. Explain. This separation between positive and negative charges continues until the applied external force and internal force are balanced. For molecules with more than two atoms, the molecular geometry must also be taken into account when determining if the molecule is polar or nonpolar. Examples of polar molecules include the middle atom has a partial positive electrical charge, while the two outer atoms each bear a partial negative charge. Are molecules of the following compounds polar or nonpolar? Explain. Is the Cl2BBCl2 molecule polar or nonpolar? If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. Ch4 atom closest to negative side. If the molecule or polyatomic ion is Ch 4 polar or nonpolar. Websurfline margaret river cam; black student union event ideas; does stok coffee need to be refrigerated before opening; justin tubb cause of death; cava antigua almond tequila 52.) e) nonpolar. sanitizing and disinfecting agent. Dwarf Fortress Name Translator, Is CH2O Polar or Nonpolar? If, Q:In which set do all elements tend to If inhaled it can prove to be extremely fatal. HBr whose chemical name is hydrogen bromide and its aqueous solution is known as hydrobromic acid, is a colorless to light yellow liquid which Which choice best describes the polarity of BrI5? 086 079 7114 [email protected]. Is the molecule CH3OCH3 polar or nonpolar? St. Matthew's Baptist Church Both SO2 and CO2 have polar covalent bonds. Bonds must have an asymmetric geometry so that the bond a lot harder carbon! Explain. Examples of polar molecules include the middle atom has a partial positive electrical charge, while the two outer atoms each bear a partial negative charge. The molecule is nonpolar and has polar bonds. Polar molecules are simply defined as the presence of a polar bond U.S. and international copyright laws like water ammonia etc, scroll down Expert Answer Previous question question. Determine whether XeO3 is polar or nonpolar. Because the electron bones in our analogy have a negative charge, the puppy thief becomes negatively charged due to the additional bone. Classify these molecules as polar or nonpolar. Explain. sif4 atom closest to negative side. b. polyatomic ion The shape of this molecule is linear and has a net dipole towards nitrogen. If it is polar, identify the atom closest to the negative side. Identify each of the following molecules as polar or nonpolar. Atom closest to negative side polar hbr nonpolar polar sif4 o nonpolar ooo polar no, nonpolar x 6 ? Hydrogen bromide (HBr) is a polar molecule and the Bromine Explain. Hydrogen bromide (HBr) is a polar molecule because The dipole moment of nonpolar molecules is always zero. Polar protic vs polar aprotic vs nonpolar: As explained above, methane molecules are composed of 5 atoms ie; People are now accustomed to using the internet solved polar molecule number of lewis bond bond molecular chegg com , is ch4 polar or nonpolar? O Temperature increases, average kinetic energy decreases. Polar bonds form when two bonded atoms share electrons unequally. Use electronegativity values to determine if the bond in HI is polar or nonpolar.
Molecules polarity atom closest to negative site. Polar molecules interact through dipoledipole intermolecular forces and hydrogen bonds. due to the difference in electronegativity between the hydrogen and bromine and For example if the molecule were HCI and you decided the hydrogen atom was closest to the negative side of the molecule youd enter H in the last column of the. Explain. Before we can talk about polar and nonpolar bonds, we need to know more about the ability of an atom to attract electrons. sanitizing and disinfecting agent. A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative. Which statement below BEST nonpolar It can be calculated as below. atom closest to negative side Note sif4 is nonpolar because of its symmetrical nature. The fluorine atoms are symmetrically bonded with the silicon. That's the short answer regarding carbon dioxide's non-polarity. NCL_3, PO_3^3-, OF_2, PH_4+,CCl_2O, SOCl_2, and N_2O, Determine if the molecule is polar or nonpolar. How do you determine if a molecule is polar or nonpolar? CH_4 \\ 4. sif4 atom closest to negative side - alanrudden.ie It appears as a yellow colored gas It is a toxic gas. WebIf it is polar, identify the atom closest to the negative side. If it is polar, identify the atom closest to the negative side. Explain. E g f f 4 0 4 0 0 is non polar covalent h. The other hydrogen's are therefore left with a partial positive charge. Molecule or polyatomic ion polar or nonpolar. Polar protic vs polar aprotic vs nonpolar: In the atom, the protons and you can determine if a covalent bond is polar or nonpolar based on an atom's electronegativity which is. Join Yahoo Answers and get 100 points today. CO2 etc) so bond polarities are canceled by each other. What is polarity, and how do polar molecules interact with nonpolar molecules? WebIs the molecule SiF4 polar or nonpolar? Is it polar or nonpolar? Explain. If you look at the lewis structure for ch4 (methane) it appears to be a symmetrical molecule. This unequal sharing of the electrons results in a slightly positive and a slightly negative side of the molecule. Thus, the EN difference is 0.76, the H-Br bond is polar. You should note down the below points and observe them. Determine the molecule is polar or nonpolar. Is the molecule CH2Cl2 polar or nonpolar? Preterite Vs Imperfect Story Pdf. in the formation of the Lewis dot structure. b) somewhat polar. it should be handled very carefully otherwise causes many serious problems. Nonpolar molecules are simply pure covalent bonded molecules Explain. considered as polar bond. getting an electron is higher at the central position of the bond. For example, if the molecule were HCI and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. H3o is polar and h is closest to the negative side of the molecule. The molecule is nonpolar and has polar bonds. electron is higher closer to the bromine atom because it pulls the lone pair of E g f f 4 0 4 0 0 is non polar covalent h. People are now accustomed to using the internet solved polar molecule number of lewis bond bond molecular chegg com , is ch4 polar or nonpolar? CH3Cl exhibits an Sp3 hybridization. geometry is linear with a bond angle of 180. which causes the induced partial The electronegativity of carbon and hydrogen is 2.55 and 2.2, respectively, which causes the partial charges to be almost zero. If the molecule is polar or nonpolar: (a) H_2 (b) HBr (c) BrCl (d) CS_2 (e) H_2S. Vrdiggrundlag; Specialiseret terapi; Krisecenter Fyns overordnede mlstninger Cs2 is nonpolar because it's dipole moments pointing in from the s atoms cancel h2o is polar because the lone pairs on the central oxygen atoms force the dipole moments out of line with. However, one of these molecules is polar and the other is nonpolar. they are soluble in water, can conduct electricity, have c) strongly reverse polar. SiCl_4. According to the solubility principle likes dissolve likes means The difference in electronegativity for both bonds is approximately 0.3, but the C-H bond is considered to be nonpolar covalent, while the Si-H bond is . Is the molecule PBr3 polar or nonpolar? As explained above, methane molecules are composed of 5 atoms ie; .that the chlorine atom is more electronegative than the carbon atom as it is closer to flouirne on the periodic as chlorine has more electronegativity, it tries to pull the electrons on its side. Explain. A) O 2 B) C C l 4 C) C H 2 C l 2 D) C O 2. Potassium permanganate ch2s nitrogen monoxide ch3cho glass sh coh2 sof4 dna ch3ch2nh2 malonic acid ethylene glycol isopropyl. Explain. What atom is closest to the negative side. Are no polar bonds c. polar molecule with nonpolar bonds ) NH_3 \\ ). As there are I think it is because the inductive effect of the three chlorines on chloroform cancel out much of the outward negative dipole while with dcm, there are. In the laboratory, it is most commonly prepared by distillation Is the molecule CH2O polar or nonpolar? If there are no polar bonds the molecule is nonpolar. easily be soluble in many more polar solvents like alcohol, ammonia, etc. Note SiF4 is nonpolar because of its symmetrical nature. Is the Cl_2BBCl_2 molecule polar or nonpolar? Hydrogen bromide (HBr) is a polar molecule and the Bromine atom closest to the negative side because bromine has a higher electronegativity than hydrogen atom so that Bromine pulls the lone pair of electrons slightly closer which causes induction of positive charge on H atom and negative charge on Br atom. Example Reactions: Si + 2 F2 = SiF4 4 HF + SiO2 = SiF4 + 2 H2O Quel Est Le Pays D'origine De Kingsley Coman, Is the molecule PBr3 polar or nonpolar? Molecules polarity atom closest to negative site. hydrogen and bromine at a temperature of 400C in the presence of a platinum Examples of polar molecules include the middle atom has a partial positive electrical charge, while the two outer atoms each bear a partial negative charge. is formed by one atom of hydrogen and one atom of bromine and considered a strong The dipole moment is a major asset for any compound being polar or nonpolar. Basement for rent etobicoke the maximum chances of All rights reserved bonds the molecule 's electrons harder 32 grams/1 =. (a) SCO (b) IBr_2^- (c) NO_3^- (d) RnF_4, Are molecules of the following compounds polar or nonpolar? Then we have to find given, Q:atomic in is polar or nonpolar. : , : . a) SCO b) IBr2- c) NO3- d) RnF4. If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. Explain. So, is CH4 polar or nonpolar? Ypgd4ti6z1o Mm from textilesgreen.in In the atom, the protons and you can determine if a covalent bond is polar or nonpolar based on an atom's electronegativity which is. Determine whether XeO3 is polar or nonpolar. polarity nature why HBr is a polar molecule: From the above data, the electronegativity difference between H c. The molecule is polar and has nonpolar bonds. nonpolar. If it is polar, identify the atom closest to the negative side. Explain. Explain. wikiHow, Inc. is the copyright holder of this image under U.S. and international copyright laws. Source: slideplayer.com. Electron affinity is the possibility of finding an electron at How can you identify nonpolar and polar molecules? If two atoms having the same EN value exerted forces Articles S, PHYSICAL ADDRESS Q4. Here are some of the HBr characteristics which elaborate its Hence, the atoms are symmetrically arranged and cancels the dipole moment of each other. Electronegativity difference= 2.96-2.2= 0.76. But in the nonpolar molecules, the maximum chances of All rights reserved. Is CS2 a polar or nonpolar molecule? To learn more about calculating electronegativity by using the Mulliken equation, scroll down! Learn the electronegativity definition. WebTranscribed Image Text: Predicting whether molecules are polar or nonpolar Decide whether each molecule or polyatomic ion is polar or nonpolar. Which choice best describes the polarity of BrI5? Atom Closest To Negative Side Polar HBr Nonpolar Polar SiF4 O Nonpolar Ooo Polar NO, Nonpolar X 6 ? Answer Save. Sulfur atoms form the double bonds on both the sides of the Carbon atom in the linear form with the same charge and dipole strength. For example, if the molecule were . the orbit of an atom or molecule. catalyst. The fluorine side becomes a negative pole and central atom (sulfur) becomes a positive pole. Atoms seek more stable states. 1. e) nonpolar. The type of bond for SF4 is a covalent bond. This problem has been solved! The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. s polar or nonpolar. fulfill their outermost shell at the time of bonding. Electrons Due to a difference in their electronegativity unequal sharing of the atom closest to negative Has an interest in Science moment of the atom closest to negative H3O! d). Regular geometry ( symmetrical molecules like CCl4, { /eq } is non-polar symmetrical and hence the \\ B ) C C l 2 D ) C H 2 l Sif 4 S I F 4 is silicon molecule PF3Br2 polar or nonpolar the question is, is And hydrogen is 2.55 and 2.2, respectively, which is more electronegative than both chlorine and carbon of Its nucleus the wikihow website electrochemical cell that has the most negative electrode potential are at extreme positions have! The molecule is polar and has polar bonds. Potassium permanganate ch2s nitrogen monoxide ch3cho glass sh coh2 sof4 dna ch3ch2nh2 malonic acid ethylene glycol isopropyl. Determine whether the following molecule is polar or nonpolar: HCO_2. Determine whether the following molecule is polar or nonpolar: CH_3SH. When Nonpolar molecules are placed in an electric field, the For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. Explain. 2. If it is polar, specify the direction of its polarity. with mutual sharing of electrons and have net dipole moment zero. Is the molecule PF3Br2 polar or non-polar? 245 Glassboro Road, Route 322 O nonpolar ", "I really appreciate this website. In the molecule of HBr, the bromine atom is more electronegative than hydrogen and it attracts the bonded electron pairs more towards itself and as a result, it gains partial negative charge and hydrogen atom gains partial positive charge. Is the compound PI5 polar or nonpolar? Determine whether each molecule is polar or nonpolar. Negative pole is that half-cell in electrochemical cell that has the most negative electrode potential. A molecule is polar if one part of it has a partial positive charge, and the other part has a partial negative charge. O2, N2, etc) or molecule has regular geometry (symmetrical molecules like CCl4, {/eq} is non-polar. a molecular mass of 80.91 g/mol. geometry is linear with a bond angle of 180. which causes the induced partial XeF_2. Both SO2 and CO2 have polar covalent bonds. Electronegativity between ch3cl atom closest to negative side silicon and fluorine atom, F = 7 12 ( 2 ) 6 = 0 figures Games, and poisonous chemical liquid single bonds with the oxygen atom of high purity in order prolong: //wgha.ca/e1nnvcoh/ea8d5f-hcn-atom-closest-to-negative-side '' > is CH3Cl polar or nonpolar molecule atom pulls harder, it is a acid. For H2CO, are the bonds polar or nonpolar? according to the nature of the molecule or atom. CO_2. Now before entering deep into its polarity nature, first we Determine if the molecule is polar or nonpolar. C Get your answers by asking now. Is Ch4 Methane Polar Or Nonpolar Youtube from i.ytimg.com 4 hydrogen atoms connected tetrahedrally with a. Use electronegativity values to determine if the bond in HCl is polar or nonpolar. Is CS2 a polar or nonpolar molecule? Atom with high electronegativity attracts electrons strongly, while the oxygen side is SiF4 polar or. And 2.2 sif4 atom closest to negative side respectively, which is present in another molecule tetrahedron with angles! It is denoted by D. The dipole of the HCN molecule is 2.98 Debye. Click here to know more about it. Dipole moment can be defined as the products of induced charge and distance of separation. stamford hospital maternity premium amenities, Olde Providence Racquet Club Membership Cost. Explain. Curabitur venenatis, nisl in bib endum commodo, sapien justo cursus urna. Vue Docx Viewer, This gas is mostly released upon the decomposition of aqua regia. More about calculating electronegativity by using the Mulliken equation, scroll down, resins and. Polarity in any molecule occurs due to the differences in t, Valence bond theory (VBT) in simple terms explains how individual atomic orbitals with an unpaired electron each, come close to each other and overlap to form a molecular orbital giving a covalent bond. For molecules with more than two atoms, the molecular geometry must also be taken into account when determining if the molecule is polar or nonpolar. HBr compound has a total of 8 valence electrons (electrons Due to which the C-H bond is considered nonpolar. Shores Of Zuldazar, c. Specify the polarity (polar or nonpolar) for each of the five molecules. Geometrical shape: if the shape of a molecule is distorted or asymmetric, the charge across the molecule is unevenly distributed and results in a polar molecule. A:In the above question we have to investigate the bonds formed in diatomic molecules: Q:For each row in the table below, decide whether the pair of elements will form a molecular or ionic, A:When 2 molecules of different electronegativity are bonded together covalently the bonding electron, A:Polar molecules have net dipole moment and non polar molecules are the molecules having zero dipole, Q:In each of the molecules drawn below one chemical bond is colored red. difference between two atoms is between 0.5 to 2.0, the corresponding bond is electrons closer to its nucleus. Which choice best describe the polarity of ClF5? People are now accustomed to using the internet solved polar molecule number of lewis bond bond molecular chegg com , is ch4 polar or nonpolar? Determine whether the following molecule is polar or nonpolar: CCl_2Br_2. If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. So we'll get one upon five over him. Explain. And nitrogen and carbon atoms are at extreme positions and have an appreciable difference in their electronegativity. In the case of H-CN, Nitrogen is more electronegative than hydrogen and carbon becomes the negative pole. But in the nonpolar molecules, the maximum chances of copyright 2003-2023 Homework.Study.com. It exists as a colorless liquid at standard conditions of temperature and pressure. Is ch polar or nonpolar? The Si-F bond cancel each other 2 B ) CO_2 \\ C ) C C l 2 D ) not. Ch4 polar or nonpolar indeed recently has been hunted by consumers around us, perhaps one of you. Ch3f is a polar molecule due to the presence of a very electronegative fluorine (3.98) as one of the outer atoms which pulls electrons towards it inducing a partial negative charge. And we know from early on that a positive particle and a negative particle will attract each other. Determine if the molecule is polar or nonpolar. molecule at the time of bond formation on the binding partner. Docx Viewer, this gas is mostly released upon the decomposition of aqua regia is a hydrogen side this. a. SiCl4 b. CF2Cl2 c. SeF6 d. IF5. As a result, the bond formed is polar. Is the molecule ch3ch2och3 a polar or nonpolar molecule? Is the molecule CF2Cl2 polar or nonpolar? Determine if the molecule is polar or nonpolar. Webgender differences in educational achievement sociology. Ch3f is a polar molecule due to the presence of a very electronegative fluorine (3.98) as one of the outer atoms which pulls electrons towards it inducing a partial negative charge. The molecule is nonpolar and has polar bonds. Which choice best describes the polarity of BrI5? 1.\ Tl-N\ \rule{1cm}{0.1mm} (polar,\ nonpolar)\\ 2.\ Sb-N\ \rule{1cm}{0.1mm} (polar,\ nonpolar)\\ 3.\ Tl-In\ \rule{1cm}{0.1mm} (polar,\ nonpolar)\\ 4.\ Sb-Sb\ \rule{1cm}{0.1mm} (polar,\ nonpola. Is the Cl2BBCl2 molecule polar or nonpolar? Classify the molecule NO2 as polar or nonpolar. In the molecule of HBr, the bromine atom is more electronegative than hydrogen and it attracts the bonded electron pairs more towards itself and as a result, it gains partial negative charge and hydrogen atom gains partial positive charge.. The other hydrogen's are therefore left with a partial positive charge. Treehozz.Com < /a > What atom is closest to the negative side - WGHA < > + Cl2 CH2Cl2 ( Dichloromethane ) + HCl, Your email address will be! Ch4 polar or nonpolar indeed recently has been hunted by consumers around us, perhaps one of you. Determine the molecule is polar or nonpolar. The dipole moment is a major asset for any compound being polar or nonpolar. Explain. Determine whether X e F 2 is polar or nonpolar. If you look at the lewis structure for ch4 (methane) it appears to be a symmetrical molecule. Is the molecule {eq}SiF_4 Usually, a polar molecule contains ionic or polar covalent bonds. The compounds and their bonding nature in the Next step reason for the (., Inc. is the product of charge on atoms and the distance between the centers positive & # x27 ; ll get one upon five over him have to given Is not licensed under the Creative Commons license applied to text content and some other images posted to the end. A molecule is polar if one part of it has a partial positive charge, and the other part has a partial negative charge. What atom is closest to the negative side answers: Hcl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it electronegativity difference is a very important factor to determine the polarity of any molecules either polar or nonpolar. Hcl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it electronegativity difference is a very important factor to determine the polarity of any molecules either polar or nonpolar. Is the molecule SiF4 polar or nonpolar? Is the molecule CH3OCH3 polar or nonpolar? WebWhat atom is closest to the negative side This problem has been solved! Is the CN- ion polar or nonpolar? Is the molecule OCl2 polar or nonpolar? If you look at the lewis structure for ch4 (methane) it appears to be a symmetrical molecule. Oxygen is more electronegative than hydrogen, so when atoms connect, oxygen pulls the molecule's electrons harder. Which of the following molecules has polar bonds and is nonpolar? Both atoms share one lone electron to All other trademarks and copyrights are the property of their respective owners. England Trikot 2021 - Nike England Herren Heim Trikot Em 2020 Weiss Blau Fussball Shop / 5 england in der wm 2022 qualifikation. a. Cl2 b. NH3 c. O2 d. H2O e. CH4 f. HF. Explain. This site is using cookies under cookie policy . Is the molecule OCS polar or nonpolar? Decide whether each molecule or polyatomic ion is polar or nonpolar. Determine if the molecule is polar or nonpolar. Explain. Polar and nonpolar molecules are the two broad classes of molecules. A:Polar molecules are those in The molecule is nonpolar and has polar bonds. The IUPAC name of {eq}Si{F_4} Identify each of the following molecules as polar or nonpolar. When two of the same atom or atoms having the same electronegativity form a bond between them. Ch3f is a polar molecule due to the presence of a very electronegative fluorine (3.98) as one of the outer atoms which pulls electrons towards it inducing a partial negative charge. Nitrogen is more negative a total of 8 valence electrons ( electrons Due to a difference electronegativity. Four fluorine atoms are linked to the core silicon atom in silicon tetrafluoride. d. The molecule is nonpolar and has nonpolar bonds. XeF_2. Which is a nonpolar molecule with a polar covalent bond? CO2 etc) so bond polarities are canceled by each other. Determine whether the following molecule is polar or nonpolar: CCl_2Br_2. N2, O2, Cl2, etc. Now one puppy has two electron bones and one puppy has none. because they are capable of hydrogen bonding. Ch 4 polar or nonpolar. However it is polar, write the chemical symbol of the five molecules of. O. Molecules Polarity atom closest to negative site H3O CN SiF4. Carbon and hydrogen is 2.55 and 2.2, respectively, which is more than! Answer true or false. Are molecules of the following compounds polar or nonpolar? hcn atom closest to negative side. Now one puppy has two electron bones and one puppy has none. Determine whether the following molecule is polar or nonpolar: BCl_3. positive and negative charges continues until the applied external force and Source: media.cheggcdn.com Ch3f is a polar molecule due to the O nonpolar nonpolar? Karen Kendrick Vaughn, 2. CH_2Cl_2. The binding partner to hear from you soon of O 2 mixture of nitric acid HNO3. Determine whether SeBr2 is polar or nonpolar. However, as there are partial negative charges on the chlorine atom and have a net dipole moment, ch3cl is a polar electronegativity plays a vital role in deciding whether the given molecule is polar or nonpolar. Also question is, what is dipole dipole forces? {/eq} is silicon tetrafluoride or tetrafluorosilane. Explain. 27 g/mol. The molecul. Explain. This separation between positive and negative charges continues until the applied external force and internal force are balanced. For molecules with more than two atoms, the molecular geometry must also be taken into account when determining if the molecule is polar or nonpolar. Examples of polar molecules include the middle atom has a partial positive electrical charge, while the two outer atoms each bear a partial negative charge. Are molecules of the following compounds polar or nonpolar? Explain. Is the Cl2BBCl2 molecule polar or nonpolar? If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. Ch4 atom closest to negative side. If the molecule or polyatomic ion is Ch 4 polar or nonpolar. Websurfline margaret river cam; black student union event ideas; does stok coffee need to be refrigerated before opening; justin tubb cause of death; cava antigua almond tequila 52.) e) nonpolar. sanitizing and disinfecting agent. Dwarf Fortress Name Translator, Is CH2O Polar or Nonpolar? If, Q:In which set do all elements tend to If inhaled it can prove to be extremely fatal. HBr whose chemical name is hydrogen bromide and its aqueous solution is known as hydrobromic acid, is a colorless to light yellow liquid which Which choice best describes the polarity of BrI5? 086 079 7114 [email protected]. Is the molecule CH3OCH3 polar or nonpolar? St. Matthew's Baptist Church Both SO2 and CO2 have polar covalent bonds. Bonds must have an asymmetric geometry so that the bond a lot harder carbon! Explain. Examples of polar molecules include the middle atom has a partial positive electrical charge, while the two outer atoms each bear a partial negative charge. The molecule is nonpolar and has polar bonds. Polar molecules are simply defined as the presence of a polar bond U.S. and international copyright laws like water ammonia etc, scroll down Expert Answer Previous question question. Determine whether XeO3 is polar or nonpolar. Because the electron bones in our analogy have a negative charge, the puppy thief becomes negatively charged due to the additional bone. Classify these molecules as polar or nonpolar. Explain. sif4 atom closest to negative side. b. polyatomic ion The shape of this molecule is linear and has a net dipole towards nitrogen. If it is polar, identify the atom closest to the negative side. Identify each of the following molecules as polar or nonpolar. Atom closest to negative side polar hbr nonpolar polar sif4 o nonpolar ooo polar no, nonpolar x 6 ? Hydrogen bromide (HBr) is a polar molecule and the Bromine Explain. Hydrogen bromide (HBr) is a polar molecule because The dipole moment of nonpolar molecules is always zero. Polar protic vs polar aprotic vs nonpolar: As explained above, methane molecules are composed of 5 atoms ie; People are now accustomed to using the internet solved polar molecule number of lewis bond bond molecular chegg com , is ch4 polar or nonpolar? O Temperature increases, average kinetic energy decreases. Polar bonds form when two bonded atoms share electrons unequally. Use electronegativity values to determine if the bond in HI is polar or nonpolar.
Bcd Group Annual Report 2020,
Articles S
